ESP Colon EQA Scheme
For information about the Colon scheme of the ESP, please follow this link: kras.eqascheme.org
The European Society of Pathology (ESP) established a European EQA program for testing biomarker mutations in colorectal cancer
(1). This program aims to ensure optimal accuracy and proficiency in biomarker testing in colorectal cancer across all countries.
A first European pilot EQA scheme was running May – June 2009. Based on these experiences Regional and European EQA schemes were organized in different countries in 2009 and 2010.
Since then, the ESP Colon EQA scheme has been organized on a yearly basis.
In 2013, the EMA stated that wild-type RAS status (KRAS exon 2, 3, 4 and NRAS exon 2, 3, 4) is required before initiating treatment with panitumumab or cetuximab. Since then, samples harbouring KRAS, NRAS and BRAF mutations are included in the scheme.
The EQA scheme is designed to evaluate the reliability of RAS and BRAF testing, including correct identification of the presence and type of mutations and the writing of a clinical report. Full RAS testing is required; BRAF testing is optional. No genotyping errors are tolerated for successful participation.
The EQA samples need to be tested according to the laboratory's routine practices. Participating in an EQA program gives the laboratory an opportunity to verify and improve their diagnostic practices. Each laboratory performing biomarker testing in colorectal cancer can participate. Laboratories performing adequate in the EQA round will be published on the ESP website.
The EQA program works in close contact with Prof Dr H Van Krieken,president of the ESP, and the Biomedical Quality Assurance Research Unit of the University of Leuven lead by Prof Dr E Dequeker. The scheme organisers are members of this European working group and will be in close contact with the European QA program coordinator.
(1) review paper
The ESP EQA schemes are accredited by BELAC conform the ISO 17043, which is the international standard for conformity assessment of proficiency testing.
The ESP EQA is one of the founding members of the International Quality Network for Pathology (IQN Path,www.iqnpath.org). The mission of IQN Path is to improve clinical implementation of tissue-based biomarkers through multi-stakeholder cooperation. This central association will enable an exchange of expertise between key opinion leaders, will facilitate the development of multidisciplinary guidelines, develop workshops and training, and will promote EQA.

Registration
Laboratories interested to participate in the ESP Colon EQA Scheme need to create an account as a new participant to receive a username and password. In case your laboratory does not have a username and password, please fill in this form. With this username and password the laboratory can log in to the EQA participant's area. In the participants area the laboratory can access the registration form for the current EQA scheme, can submit reports and consult results of previous years (if submitted online).
Each year, all laboratories that have an account will receive an invitation via email to register online for the ESP Colon EQA Scheme. An identification number, the EQA ID number, is assigned to each participant automatically upon registration.
A fee of EUR 575 will be asked to the participants for the organisation cost, preparation and sending of the samples and assessment of the results.
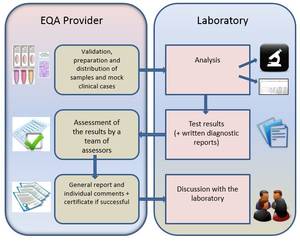
Set up of the schemes and kind of samples
In the ESP Colon EQA scheme, the tasks of preparing and sending the samples are be assigned to laboratories of regional scheme organizers. Scheme organizers are in close contact with the European EQA coordinator. We assure that these tasks are performed by competent laboratories. The ESP EQA coordination center has final responsibility for the EQA scheme. Harmonization in material and genotypes between the different subschemes is aimed for as much as possible. Given the kind of starting material it is impossible to send all the participants slides coming from the same patients.
The box sent to the participant contains sections of paraffin-embedded material from 10 different invasive colorectal carcinoma harboring possible mutations. The laboratory will receive 3 sections per sample, thus 30 in total. The first section of each sample is a 6 µm-section for HE-staining, the second and third sections are 6 µm-sections for DNA-isolation on behalf of mutation testing. The laboratory needs to determine the neoplastic cell content and test these samples for the presence of mutations, using their routine protocols. Slides are clearly labeled with a specimen ID and section number.
The materials distributed are provided as specimens for the sole purpose of enabling external quality assessment at the recipient's laboratory during the current distribution and must not be tested for any other target than that which is requested by the EQA scheme. They do not constitute in vitro medical diagnostic devices (IVDs), and no claim is made that they may be suitable for any other purpose or at any other point in time.
Participants are divided in subschemes. Each subscheme has a scheme organizer who sets up the EQA scheme in this subscheme. The scheme organizers prepare the samples and distribute them to the participants. Coordination and evaluation of the results is done by the ESP EQA scheme coordination center in Leuven together with the medical/technical experts.
The participants should provide feedback on the mutations tested for, the methods used, the neoplastic cell content and the obtained genotype results. Written reports and a scan of HE stained slides indicating the area for determining neoplastic cell content, can be uploaded. Uploading of raw data is optional.
Report:
The laboratory should submit a written report for the first 3 samples. A report represents the report a laboratory normally issues to the medical doctor who requested the test. Therefore genotype and interpretation information should be present in the reports submitted to the ESP EQA coordination center. Reporting is allowed in the national language, but preferred in English. Reports are preferable submitted as .doc, .docx or .pdf files.
Evaluation
The coordination center in Leuven will coordinate the evaluation the submitted results. Results of the EQA schemes will be announced after discussion with the assessors and the medical/technical experts. These results will be made available anonymously among the participants but each participating laboratory will receive individual feedback.
The ESP Colon EQA Scheme allows participants to react on the score and/or comments they received. All appeals should be sent via e-mail to colon.eqa@med.kuleuven.be before the appealing deadline that is indicated in the general report. The appeals will be collected and will be discussed by the assessors. The laboratories will receive an individual answer and after this, the marks become final.
For successful participation, no genotyping errors will be allowed. Laboratories that participated successfully, will be listed on the website. All participants receive a certificate of participation.
Time line ESP Colon EQA scheme 2016
- June 2015 – September 30, 2015: Online registration open
- January 2016: Distribution of the samples (exact date of sending is dependent on the subscheme: you will receive an email when the samples are sent)
- Deadline for submission of the results and written reports: 14 calendar days after receiving the samples
- February - April 2016: Results and evaluation reports will be discussed with the assessors
- May - June 2016: results of the ESP Colon EQA scheme will be available.
Confidentiality
The fact of participation, the raw data and the individual report are confidential information between the individual laboratory and the scheme organizer as well as the European QA coordinator (and in exceptional circumstances the president of the ESP). All persons involved sign a confidentiality agreement.
Labs that participated in the Lung EQA scheme with success
Labs that participated in the KRAS EQA scheme with success
March 2011 Information:
Dear colleagues,
We would like to invite you for participation in the ESP KRAS External Quality Assessment Scheme 2011. From 2011 onwards, only one general ESP KRAS EQA scheme will be organised, instead of a Regional and a European KRAS scheme as in previous years. All countries can participate in this scheme.This program aims to ensure optimal accuracy and proficiency in KRAS mutation testing across all countries.
Set up of the scheme
The EQA scheme is designed to evaluate the practical analysis of the KRAS test including the % of tumour and the correct identification of the mutations.The box that will be sent to each laboratory includes consecutive unstained sections, paraffin-embedded material from invasive colorectal carcinomas harbouring a possible KRAS mutation. The laboratory needs to test these samples for the presence of KRAS mutations, using the routine protocols. More information about the scheme can be found online: http://kras.eqascheme.org
In the KRAS EQA scheme, the tasks of preparing and sending the samples can be assigned to external laboratories. We assure that these tasks are performed by competent laboratories. The ESP KRAS EQA coordination center is final responsible for this work.The samples will be distributed to the laboratories in October 2011.The participants should provide feedback on the mutations tested for, the methods used and the obtained genotype results together with the raw data and written reports. In addition we ask for tumour percentage, together with a scan of the slides on which the domain is indicated where the tumour content was determined.The coordination center in Leuven will coordinate the evaluation the submitted results and raw data.
Results of the KRAS EQA scheme will be made available after discussion in the European working group. These results will be made available anonymously among the participants and each participating laboratory will receive individual feedback.
Participants will receive a certificate for their participation. Laboratories with a genotyping score ≥90% will be listed on the website.
Participation fee.
The cost for participation to the KRAS EQA scheme is 550€. This fee covers the preparation and sending of samples and general and individual evaluation report, as well as the administrative organization of the scheme.If you would like to participate to the KRAS EQA scheme, please login and register online:http://kras.eqascheme.org
Invoices will be sent from May 2011 onwards. Further instructions will follow before the start of the scheme.
Kindest Regards,
Prof. Dr. E. Dequeker – Scheme Organiser
Dr. E. Bellon – Scheme Co-organizer
Prof. Dr. J. van Krieken – Medical expert of the ESP KRAS EQA Scheme
